Novel therapeutic targets for dementia
Description
Dementia is a mental health condition that affects memory, reasoning, perception and communication. More than 850,000 people live with dementia in the UK and 60% of the cases are caused by Alzheimer’s disease (AD). Dementia costs society £26 billion per year in the UK and constitutes one of the most important health problems for the ageing population. While significant efforts have been devoted to understand multiple genetic causes and pathological mechanisms of AD, no treatments have been able to date to stop or reverse its progression. Importantly, the shape and connectivity of neurons are progressively affected by AD, and together with the accumulation of Abeta peptide and abnormal protein Tau found in post-mortem brains, they are main clues to try to understand what causes AD and how the brain is damaged in AD and other neurodegenerative conditions.
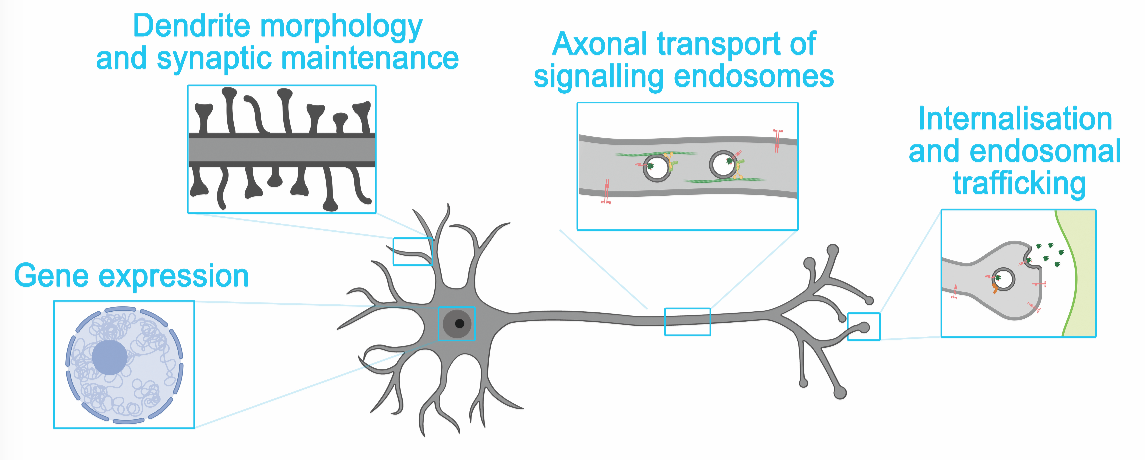
Since survival, shape and connectivity of many types of brain cells largely depend on a family of molecules called neurotrophins, these factors have been proposed to play a key role in neurodegenerative diseases and are primary therapeutic targets in AD. One of the most important members of the neurotrophins family is the brain-derived neurotrophic factor (BDNF), which binds to a receptor called TrkB on the surface of neurons. Upon BDNF binding, this receptor is internalised to generate intracellular signals with extraordinary spatial and temporal precision. BDNF/TrkB signalling is decreased in AD patients and animal models of AD. Furthermore, several therapies involving reprogramming of brain cells to produce BDNF or drugs that mimic its mode of action have shown promising results and are being tested in clinical trials.
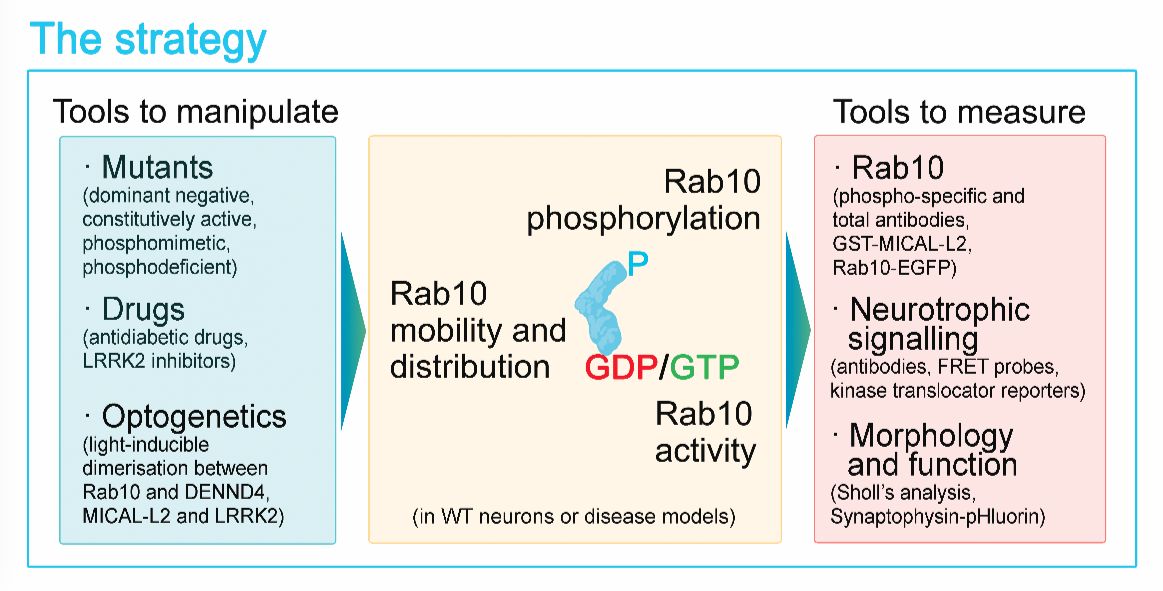
The big challenge still to be addressed is bringing the signal generated by neurotrophins to the specific sites where it is required. When BDNF is secreted by a neuron, axon terminals of other connected neurons are exposed to the neurotrophin, causing the activation, internalisation and transport of TrkB receptors along the axon towards the cell body. The arrival of the activated receptors in the cell body triggers signalling cascades that regulate shape, connectivity and gene expression. Recent results from our laboratory indicate that the small GTPase Rab10 is crucial for the sorting of internalised TrkB receptors to long-range axonal transport. Interestingly, Rab10 has been found concentrated at sites of accumulation of the protein Tau and around senile plaques in post-mortem brains of AD patients. Furthermore, a recent whole genome study revealed that in families carrying the ApoE epsilon4 allele, which increase the risk of AD, a rare variant of the Rab10 gene confers protection against dementia.
This project has been designed to determine whether the upregulation of Rab10 activity in neurons can overcome the trafficking impairments that characterise AD and restore normal neurotrophic signalling. Here, we propose to manipulate the activity of Rab10 with high spatial and temporal precision by using light-inducible probes in AD neurons both in vitro and in vivo to test whether Rab10 modulation can protect neurons from the disease. Since Rab10 is also a target of anti- diabetic drugs that are already available on the market, we aim to carry out a pilot study to determine whether increasing pharmacologically Rab10 function in AD neurons and mice improves disease outcome, therefore accelerating the translation of our pre-clinical research into new therapies tackling dementia.
Where
The Molecular NeuroPathoBiology Lab, UCL Institute of Neurology.
UK Dementia Research Institute.